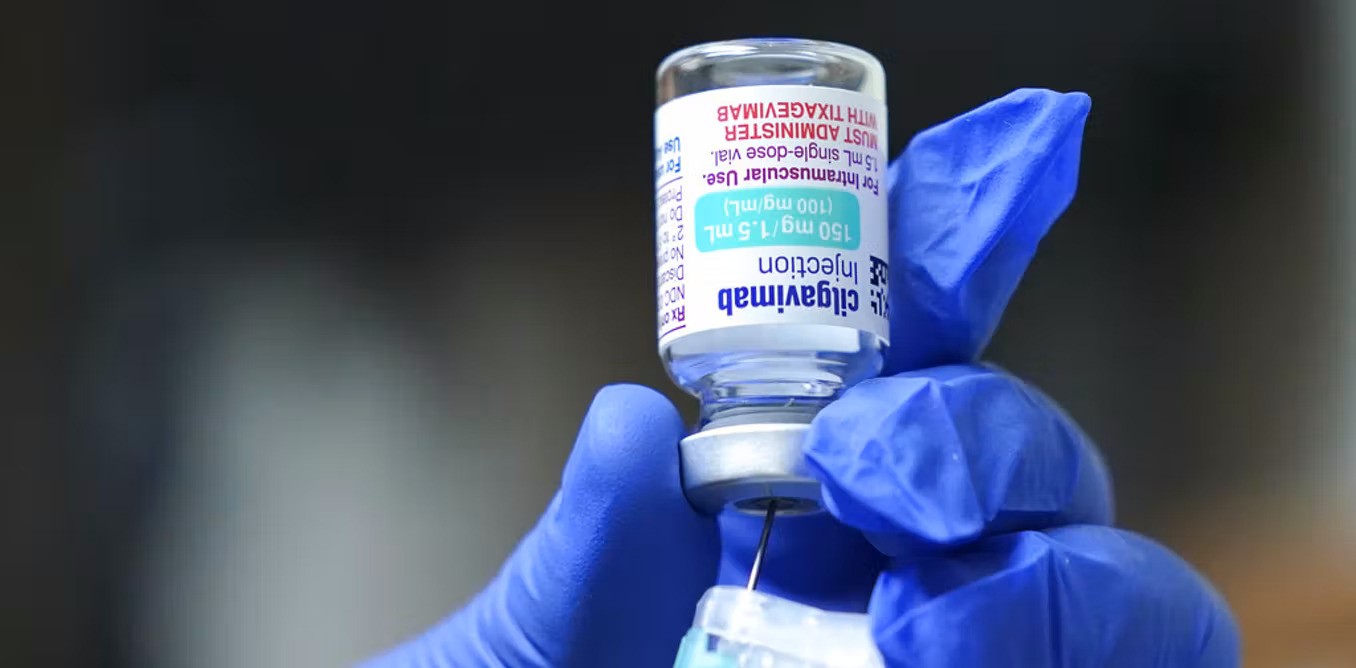
Evusheld was approved by the American Food and Drug Administration (FDA) to be used to prevent COVID-19 infections.
Customs administration in southern China's Haikou finished a special import examination and approval of the first batch of Evusheld developed by the Cambridge-headquartered company, ChinaNews.Com reported today. The batch is worth CNY22 million ($3.3 million).
Evusheld was approved to be sold in the United States late last year as well as in Europe this March. The drug contains monoclonal antibodies that can help the immune system fight the virus. It is not a substitute for vaccination. Evusheld is mostly used for immunocompromised adults and youth aged 12 or above. It should be effective for six months.
The drug will be used in experiments in hospitals in Hainan province’s Boao Lecheng International Medical Tourism Pilot Zone, according to the report. Formed in 2013, the pilot zone is designed for licensed medical treatment and clinic research. It is an important part of the Hainan Free Trade Port.
Source: Yicai Global