Médecins Sans Frontières/Doctors Without Borders (MSF) has called on Johnson & Johnson (J&J) to abandon its extended patents on lifesaving TB drug. MSF also urged countries with a high TB burden to apply a compulsory licensing mechanism to provide people with the more affordable treatment they need to stay alive and healthy.
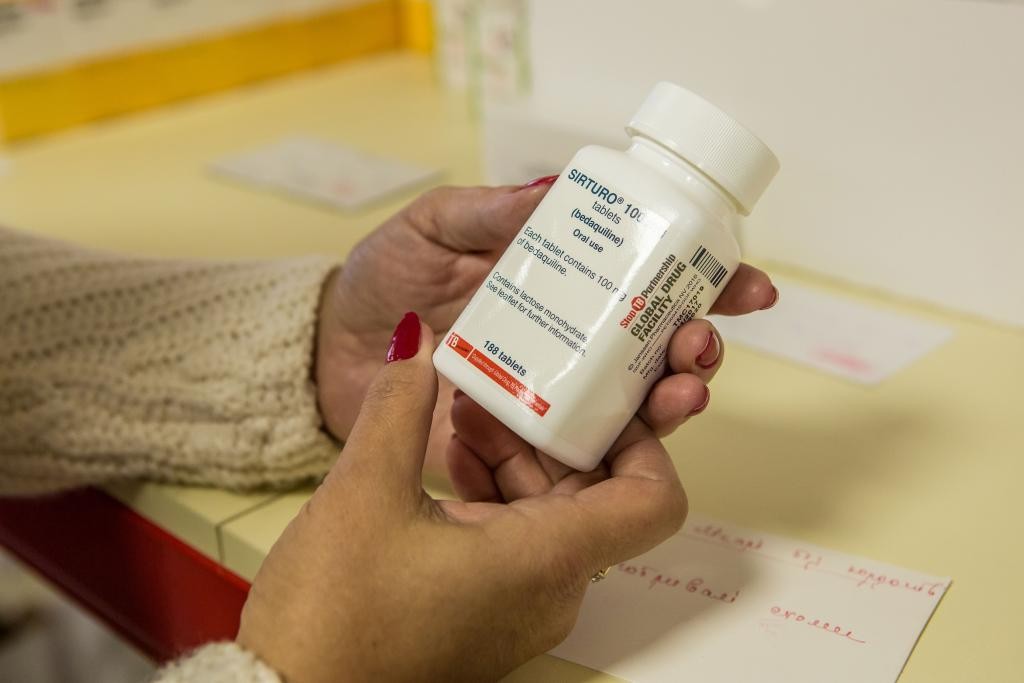
Johnson & Johnson’s 20-year primary patent on the critical, lifesaving drug-resistant tuberculosis (DR-TB) drug bedaquiline expired on July 18 in most countries, including India.
MSF reiterated its call for the US pharmaceutical corporation to publicly announce it will not enforce any 'secondary' patents for the drug in any country with a high burden of TB, and withdraw and abandon all pending secondary patent applications for this critical drug everywhere.
According to WHO, 90% of annual new TB cases occur in 30 countries with a heavy TB burden.
In July 2023, the patent on the main basic compound expired, but the owning company decided to resort to the "evergreen patent" mechanism by filing a patent application for the invention (fumarate salt). Thus, in countries where this patent has been granted, J&J has extended its monopoly on the drug until 2027, preventing alternative suppliers from entering the market and blocking access to the drug.
MSF called for a commitment from J&J to not take any legal action against any generic manufacturer that exports generic versions of bedaquiline to or from TB high-burden countries where secondary patents on the drug exist.
In mid-July, following lengthy negotiations, Johnson & Johnson has granted Stop TB Partnership´s Global Drug Facility`s licenses that enable Global Drug Facility (GDF) to tender, procure, and supply generic versions of SIRTURO® (bedaquiline) for the majority of low-and middle-income countries, including countries where patents remain in effect.
MSF noted that the GDF's deal with J&J to increase access to affordable generic versions of bedaquiline excludes many countries that have a high burden of people living with TB, primarily in Eastern Europe and Central Asia (EECA).
J&J holds secondary patents in at least 34 of the 49 countries with a high burden of TB, TB-HIV and/or DR-TB, for which bedaquiline is an essential part of treatment regimens. J&J had attempted to extend its patent by 4 years in India by filing for a secondary patent, this request was rejected by the Indian Patent Office in March 2023.
With the primary patent expiring in India today, multiple manufacturers will now be able to produce and sell generic versions of the drug freely in India and export them to any other country where patents do not stand in the way.
The current recommended by the WHO treatment, containing bedaquiline, is all oral, 6 months long and can achieve cure rates as high as 89%. This is a vast improvement over the older treatments that had to be administered for 18 months and included daily painful injections.
MSF urged any country where J&J has patents on bedaquiline, and is excluded from the GDF deal, to issuing 'compulsory licenses,' which allow producers other than the patent holder to make and sell the drug, or for more affordable generic versions of the drug to be imported even if a patent is in place.
The introduction of compulsory licensing to protect the public interest, including public health, is stipulated by the World Trade Organization (WTO) rules, outlined in the 'TRIPS' Agreement and Doha declaration.
Source: The Hindu